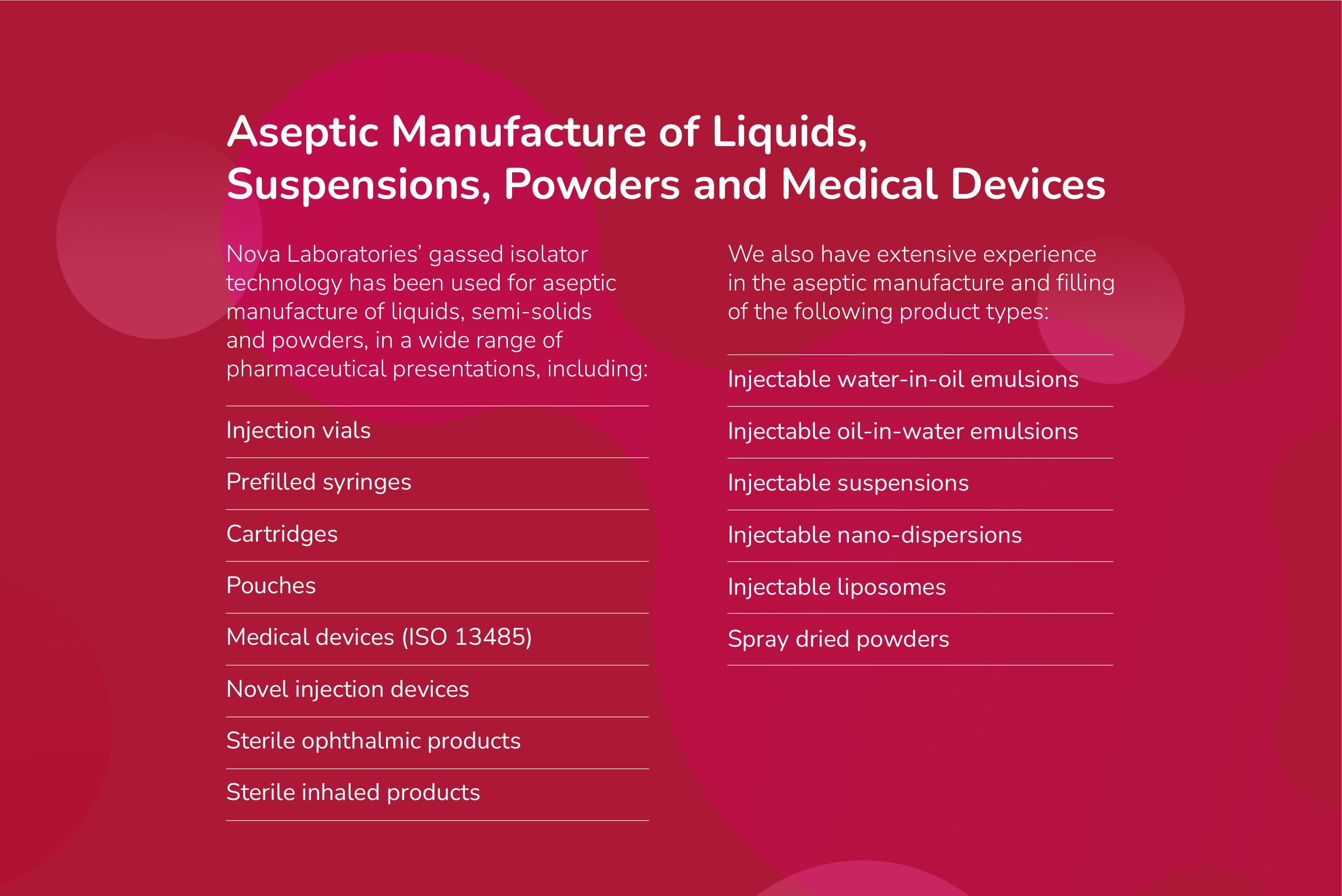
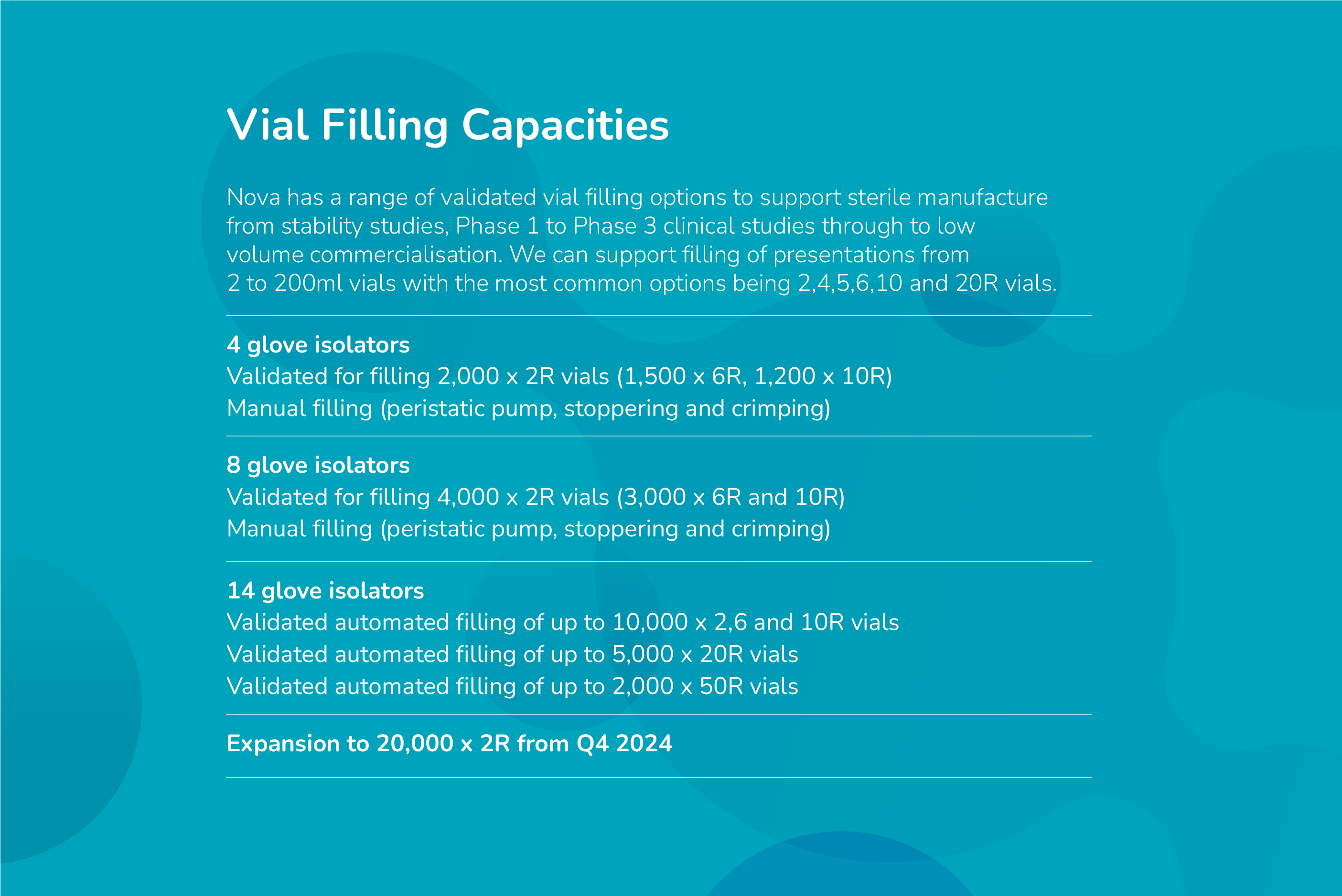
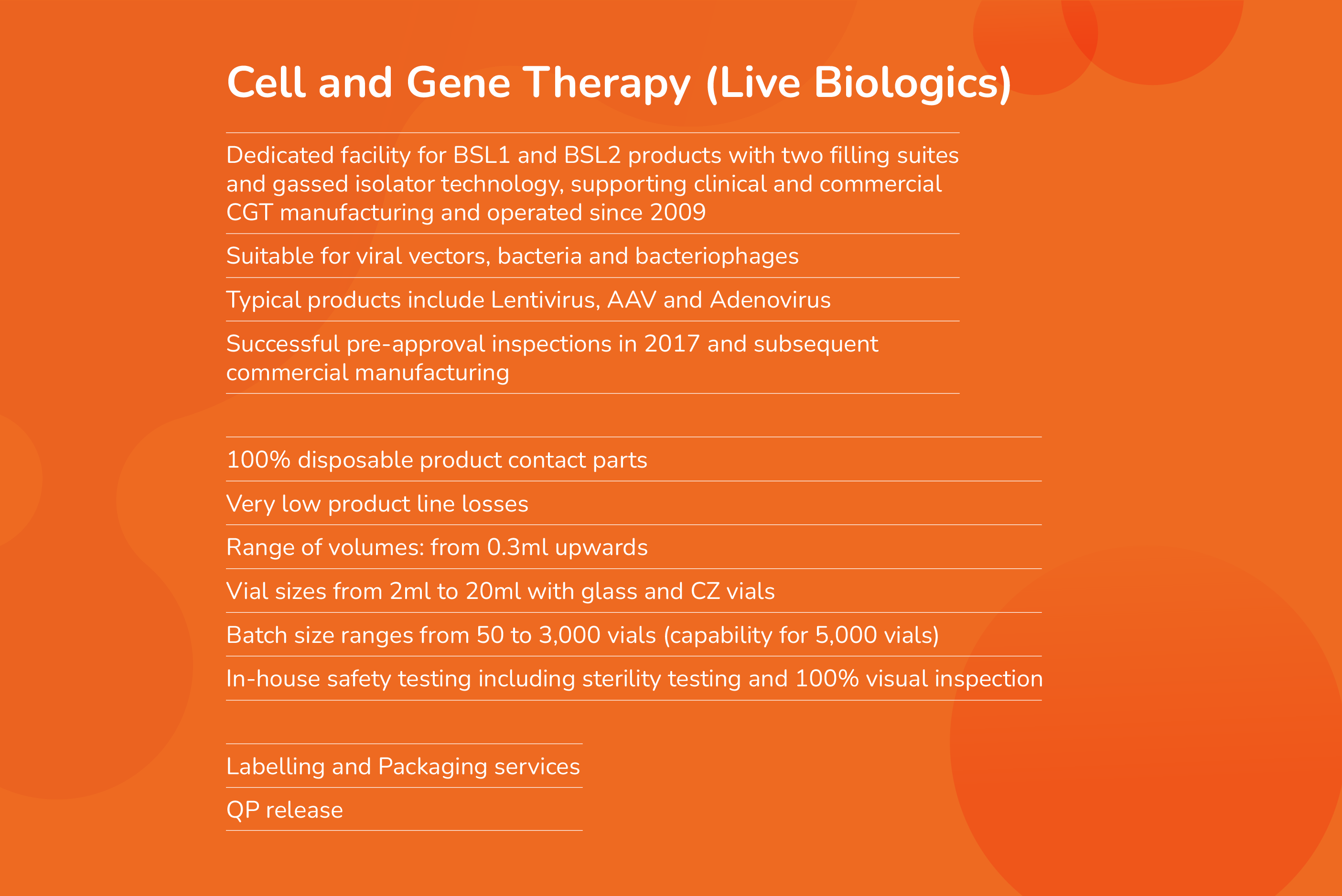
Nova Licensed Products 
With in-house clinical expertise we have in-depth knowledge of current deficiencies in the treatment of rarer diseases, with a particular focus on creating liquid versions of existing medicines for precision dosing and use in paediatrics. Our formulation team have successfully created stable, liquid versions of several existing medicines, now brought to market, and continue to work on our pipeline.
Manufactured using our own equipment and facilities, we control all aspects from development batches to the scale-up of commercial batch size, QC testing, labelling and packaging, and QP release. Stored onsite in temperature-controlled facilities, we supply directly to NHS hospitals and GB community pharmacies, and via our distribution partners for territories outside of GB. For territories in the EU, we have our sister company, Nova Laboratories Ireland, for EU import testing and QP release before distribution within the EU community.
For more information on our licensed products, head to our Knowledge Centre